Cyclomydril: Package Insert / Prescribing Info
Package insert / product label
Generic name: cyclopentolate hydrochloride and phenylephrine hydrochloride
Dosage form: ophthalmic solution
Drug class: Mydriatics
Medically reviewed by Drugs.com. Last updated on May 26, 2025.
On This Page
Cyclomydril Description
CYCLOMYDRIL® (cyclopentolate hydrochloride and phenylephrine hydrochloride ophthalmic solution) is a mydriatic prepared as a sterile topical ophthalmic solution. The active ingredients are represented by the chemical structures:
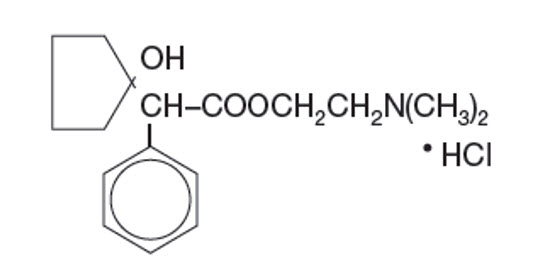
Established Name:
Cyclopentolate Hydrochloride
Chemical Name:
2-(Dimethylamino)ethyl 1 - hydroxy-α-phenylcyclopentaneacetate hydrochloride)
Molecular Formula:
C17H25NO3 • HCl
Molecular Weight:
327.85 g/mol
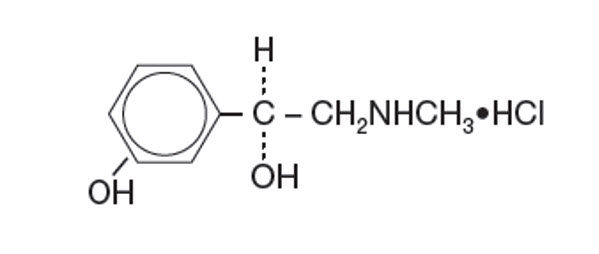
Established Name:
Phenylephrine Hydrochloride
Chemical Name:
3-hydroxy-α[(methylamino)-methyl]-, Benzenemethanol, hydrochloride (R)-.
Molecular Formula:
C9H13NO2 • HCl
Molecular Weight:
203.67 g/mol
Each mL of CYCLOMYDRIL® (cyclopentolate hydrochloride and phenylephrine hydrochloride ophthalmic solution) contains:
Active: cyclopentolate hydrochloride 0.2%, phenylephrine hydrochloride 1%. Preservative: benzalkonium chloride 0.01%.
Inactives: edetate disodium, boric acid, hydrochloric acid and /or sodium carbonate (to adjust pH), purified water.
Cyclomydril - Clinical Pharmacology
Cyclopentolate hydrochloride is an anticholinergic drug and phenylephrine hydrochloride is an adrenergic drug. This combination induces mydriasis that is greater than that of either drug alone at its respective concentration. The concentrations of cyclopentolate hydrochloride and phenylephrine hydrochloride have been selected to induce mydriasis with little accompanying cycloplegia. Heavily pigmented irides may require more doses than lightly pigmented irides.
Contraindications
Do not use in patients with hypersensitivity to any component of this preparation.
Warnings
FOR TOPICAL OPHTHALMIC USE ONLY. NOT FOR INJECTION. There have been reports of serious cardiovascular reactions, including ventricular arrhythmias and myocardial infarctions, in patients using higher concentrations of phenylephrine. These episodes, some fatal, have usually occurred in patients with pre-existing cardiovascular diseases. This preparation may cause central nervous system (CNS) disturbances. This is especially true in younger age groups, but may occur at any age, especially with the stronger solutions. Infants are especially prone to CNS and cardiopulmonary side effects from cyclopentolate.
Precautions
General
Caution should be observed when considering use of this medication in the presence of Down's syndrome due to potential increased sensitivity to anticholinergics. This product should be used with caution in patients at risk for angle closure due to the potential to precipitate acute angle closure. Because of the risk of provoking hyperthermia, use with caution in patients, especially children, who may be exposed to elevated environmental temperatures or who are febrile. The use of phenylephrine in the eye may liberate pigment granules from the iris. Mydriatics may produce a transient elevation of intraocular pressure.
Information for Patients
Do not touch dropper tip to any surface, as this may contaminate the solution. Patient should be advised not to drive or engage in hazardous activities while pupils are dilated. Patient may experience sensitivity to light.
Carcinogenesis, Mutagenesis, Impairment of Fertility
There have been no long-term studies done using cyclopentolate hydrochloride and/or phenylephrine hydrochloride in animals to evaluate carcinogenic potential.
Pregnancy
Animal reproduction studies have not been conducted with cyclopentolate hydrochloride and/or phenylephrine hydrochloride. It is also not known whether cyclopentolate hydrochloride and/or phenylephrine hydrochloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. CYCLOMYDRIL® (cyclopentolate hydrochloride and phenylephrine hydrochloride ophthalmic solution) should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether these drugs are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when CYCLOMYDRIL® (cyclopentolate hydrochloride and phenylephrine hydrochloride ophthalmic solution) is administered to a nursing woman.
Pediatric Use
Use of cyclopentolate has been associated with psychotic reactions and behavioral disturbances in pediatric patients. Increased susceptibility to cyclopentolate has been reported in infants, young children, and in children with spastic paralysis or brain damage. These disturbances include ataxia, incoherent speech, restlessness, hallucinations, hyperactivity, seizures, disorientation as to time and place. Feeding intolerance may follow ophthalmic use of this product in infants. It is recommended that feeding be withheld after administration, and to observe infants closely for at least 30 minutes (see PRECAUTIONS).
Adverse Reactions/Side Effects
Ocular
The following ocular adverse experiences have been associated with the use of CYCLOMYDRIL® (cyclopentolate hydrochloride and phenylephrine hydrochloride ophthalmic solution): increased intraocular pressure, burning/irritation upon instillation, photophobia, blurred vision and superficial punctate keratitis.
Nonocular
Use of cyclopentolate hydrochloride has been associated with psychotic reactions and behavioral disturbances in children. These disturbances include ataxia, incoherent speech, restlessness, hallucinations, hyperactivity, seizures, disorientation as to time and place. This drug produces reactions similar to those of other adrenergic and anticholinergic drugs; however, the central nervous system manifestations as noted above are most common. Other manifestations of adrenergic and anticholinergic topical ophthalmic drugs include tachycardia, hyperpyrexia, hypertension, vasodilation, urinary retention, diminished gastrointestinal motility, convulsion, bradycardia, apnea, necrotizing colitis and abdominal distention (in newborns and especially premature infants), skin rash, drowsiness, and decreased secretion in salivary and sweat glands, pharynx, bronchi and nasal passages. Severe manifestations of toxicity include coma, medullary paralysis and death. Systemic toxicity can result from topical application of sympathomimetic drugs: headache, blood pressure elevation, extrasystoles, tachycardia, syncope and cerebrovascular accidents have been reported.
Related/similar drugs
Tropicacyl
Tropicacyl is used for pupillary dilation, refraction, assessment
Cyclogyl
Cyclogyl is used for pupillary dilation, refraction, assessment, uveitis
Nurtec ODT
Nurtec ODT (rimegepant) is used to treat acute migraines and prevent episodic migraines, by ...
Mydriacyl
Mydriacyl is used for pupillary dilation, refraction, assessment
Cyclopentolate ophthalmic
Cyclopentolate ophthalmic is used for pupillary dilation, refraction, assessment, uveitis
Tropicamide ophthalmic
Tropicamide ophthalmic is used for organophosphate poisoning, pupillary dilation, refraction, assessment
Phenylephrine ophthalmic
Phenylephrine ophthalmic is used for eye redness, pupillary dilation
Atropine ophthalmic
Atropine ophthalmic is used for pupillary dilation, refraction, assessment, uveitis
Scopolamine ophthalmic
Scopolamine ophthalmic is used for pupillary dilation
Overdosage
Excessive dosage may produce behavioral disturbances, tachycardia, hyperpyrexia, hypertension, elevated intraocular pressure, vasodilation, urinary retention, diminished gastrointestinal motility and decreased secretion in salivary and sweat glands, pharynx, bronchi and nasal passages. Pulmonary oedema or cardiac arrest may occur due to phenylephrine toxicity. Severe intoxication is characterized by central nervous system depression, coma, circulatory and respiratory failure, and death.
Patients exhibiting signs of overdosage should receive supportive care and monitoring.
Cyclomydril Dosage and Administration
Instill one drop in each eye every five to ten minutes. To minimize systemic absorption, apply pressure over the nasolacrimal sac for two to three minutes following instillation. Observe infants closely for at least 30 minutes.
How is Cyclomydril supplied
CYCLOMYDRIL® (cyclopentolate hydrochloride and phenylephrine hydrochloride ophthalmic solution) is supplied as a sterile solution in 2 mL and 5 mL, in plastic DROP-TAINER® dispensers.
2 mL NDC 0065-0359-02
5 mL NDC 0065-0359-05
Storage: Store at 8°C to 25°C (46°F - 77°F)
Distributed by:
ALCON LABORATORIES, INC.
6201 South Freeway
Fort Worth, Texas 76134-2099
© 2025 Alcon Inc.
Revised: May 2025.
300065206-1223
PRINCIPAL DISPLAY PANEL
NDC 0065-0359-05
Alcon
Cyclomydril®
(cyclopentolate
hydrochloride,
phenylephrine
hydrochloride
ophthalmic
solution)
5 mL Sterile
Rx Only
Sterile Ophthalmic Solution
USUAL DOSAGE: Instill one drop
in each eye every five to ten
minutes. Read enclosed insert.
FOR TOPICAL OPHTHALMIC USE
ONLY
WARNING: Do not touch
dropper tip to any surface as
this may contaminate the
solution.
INGREDIENTS: Each mL
contains: Active:
cyclopentolate hydrochloride
0.2%, phenylephrine
hydrochloride 1%.
Preservative: benzalkonium
chloride 0.01%. Inactives:
edetate disodium, boric acid,
hydrochloric acid and/or
sodium carbonate (to adjust
pH), purified water.
STORAGE: Store at 8°-25° C
(46° to 77° F).
Alcon
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
Printed in USA
SN:
LOT:
EXP.:
GTIN: 00300650359054
300048632-0821
NDC 0065-0359-05
Alcon
Cyclomydril®
(cyclopentolate
hydrochloride,
phenylephrine
hydrochloride
ophthalmic
solution)
Sterile 5 mL
Rx Only
INGREDIENTS: Each mL contains: Actives: cyclopentolate
hydrochloride 0.2%, phenylephrine hydrochloride 1%.
Preservative: benzalkonium chloride 0.01%. Inactives:
edetate disodium, boric acid, hydrochloric acid and/or
sodium carbonate (to adjust pH), purified water.
WARNING: Do not touch dropper tip to any surface as this
may contaminate the solution.
USUAL DOSAGE: Instill one drop in each eye every five to
ten minutes. Read enclosed insert.
STORAGE: Store at 8°-25° C (46° to 77° F).
Printed in USA
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
LOT:
EXP.:
300048631-0821

| CYCLOMYDRIL
cyclopentolate hydrochloride and phenylephrine hydrochloride solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Alcon Laboratories, Inc. (008018525) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Alcon Research LLC | 007672236 | manufacture(0065-0359) | |
More about Cyclomydril (cyclopentolate / phenylephrine ophthalmic)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- Dosage information
- Drug class: mydriatics
- En español
