Nitro-Time: Package Insert / Prescribing Info
Package insert / product label
Generic name: nitroglycerin
Dosage form: extended-release capsules
Drug classes: Antianginal agents, Vasodilators
Medically reviewed by Drugs.com. Last updated on Mar 3, 2025.
On This Page
How is Nitro-Time supplied
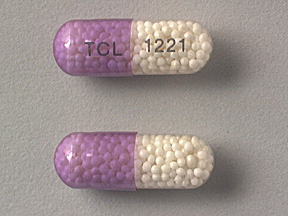
2.5 mg Nitroglycerin Extended-Release (Pink and Clear Capsules imprinted TCL-1221) in bottles of 60's & 100's
How is Nitro-Time supplied
6.5 mg Nitroglycerin Extended-Release Capsules Blue/Yellow imprinted TCL1222 in bottles of 60's and 100's
How is Nitro-Time supplied
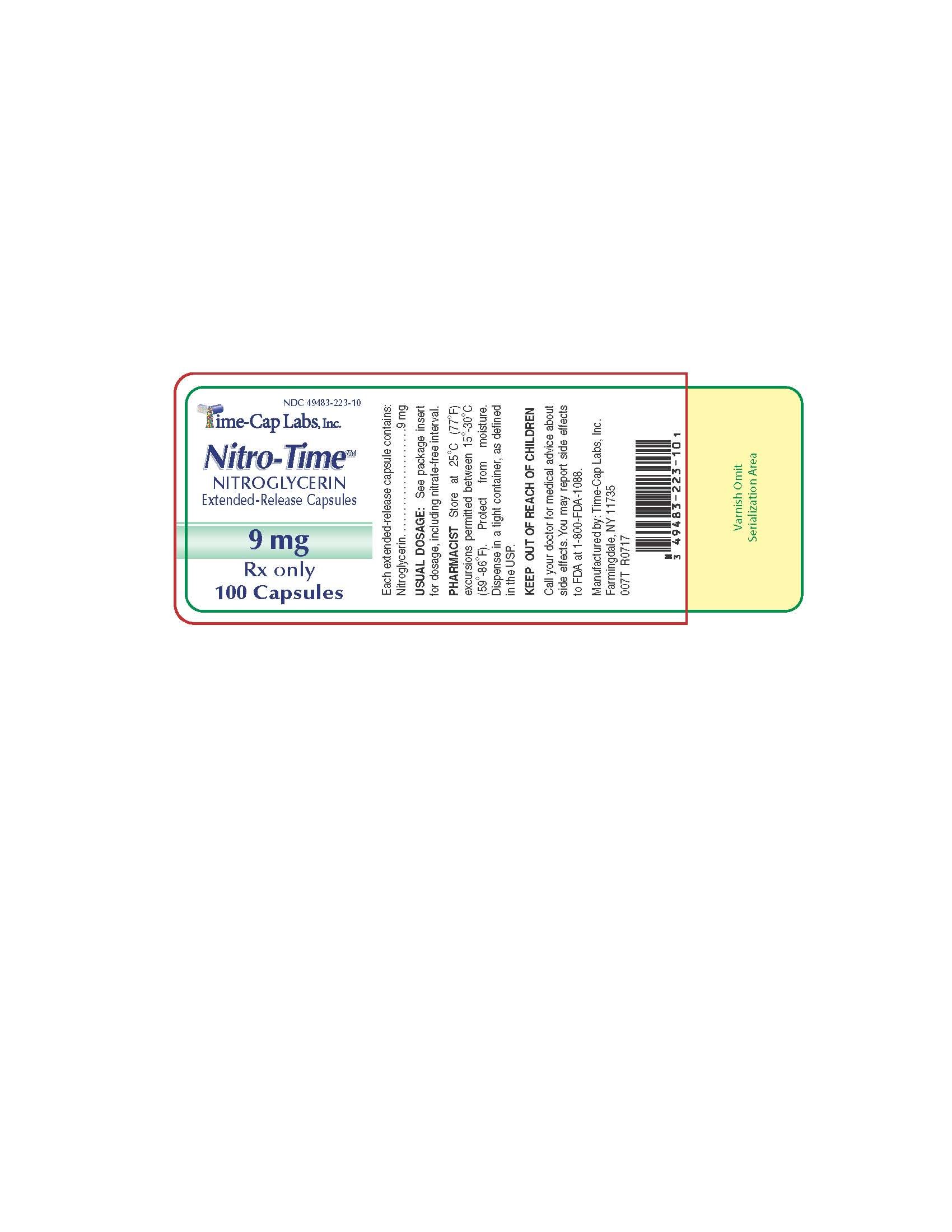
9.0 mg Nitroglycerin Extended Release Capsules Green/Yellow imprinted TCL1223 in bottles of 60's and 100's
Related/similar drugs
Clopidogrel
Clopidogrel systemic is used for acute coronary syndrome, acute coronary syndrome, prophylaxis ...
Propranolol
Propranolol is a beta-blocker that is used to treat tremors, chest pain, high blood pressure, heart ...
Ozempic
Learn about Ozempic (semaglutide) for type 2 diabetes treatment, weight management, cardiovascular ...
Spironolactone
Spironolactone is a potassium-sparing diuretic that is primarily used to treat heart failure, high ...
Hydrochlorothiazide
HCTZ (hydrochlorothiazide) used to treat high blood pressure (hypertension) and edema. Includes ...
Furosemide
Furosemide is a loop diuretic used to treat fluid retention and high blood pressure by increasing ...
Carvedilol
Carvedilol (Coreg) is used to treat heart failure and hypertension (high blood pressure). Includes ...
Aspirin
Aspirin is used to treat mild to moderate pain and to reduce fever or inflammation. Learn about ...
Metoprolol
Metoprolol is used to treat angina (chest pain) and hypertension (high blood pressure). Learn about ...
NITROGLYCERIN 2.5 MG 100 COUNT LABEL

| NITRO-TIME
nitroglycerin capsule |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| NITRO-TIME
nitroglycerin capsule |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| NITRO-TIME
nitroglycerin capsule |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - TIME CAP LABORATORIES, INC (037052099) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TIME CAP LABORATORIES, INC | 037052099 | manufacture(49483-221, 49483-222, 49483-223) | |
Frequently asked questions
- How do you take GoNitro to treat an angina attack (chest pain)?
- What is the shelf life of nitroglycerin tablets?
More about Nitro-Time (nitroglycerin)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: antianginal agents
- Breastfeeding
- En español
Patient resources
Professional resources
Other brands
Nitrostat, Nitro-Dur, Nitro-Bid, Nitrolingual Pumpspray, ... +4 more